RELATED PUBLICATIONS
Overcoming resistance with fast‑acting solid forms of deltamethrin
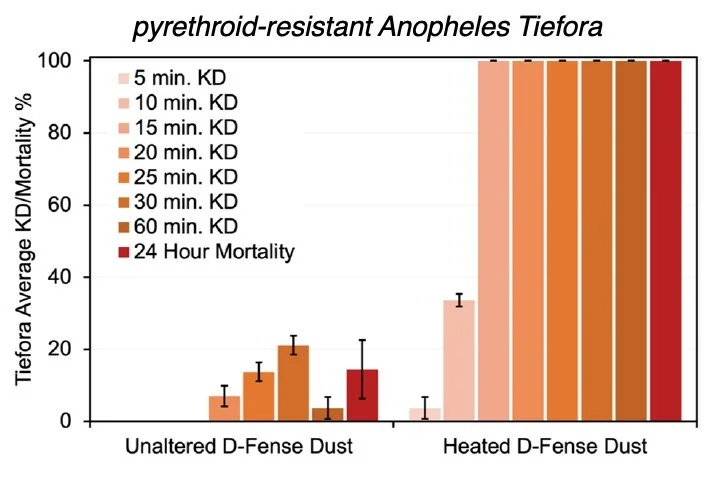
Commercial deltamethrin dust that is heat-activated to generate the more active deltamethrin polymorph (form II) was substantially more effective than the commercially available formulation used as obtained. 100% of both Banfora M and Kisumu populations were knocked down 10 min post-exposure with no recovery afterwards. Gaoua-ara and Tiefora strains exhibited 100% knockdown within 15 min, and the VK7 2014 strain exhibited 100% knockdown within 20 min. In all cases, 100% mortality was observed 24 h post-exposure. The heat-activated dust maintained comparable efficacy 13 months after heating. This reveals that higher energy polymorphs of commonly used insecticides can be employed to overcome various resistance mechanisms in African Anopheles mosquitoes through more rapid uptake of insecticide molecules from their respective solid surfaces. That is, resistant mosquitoes can be killed with an insecticide to which they are resistant without altering the molecular composition of the insecticide.
Read the article here.
J. Carson, B. Erriah, S. Herodotou, A. Shtukenberg, L. Smith, S. Ryazanskaya, M. D. Ward, B. Kahr, R. S. Lees, Malaria Journal 2023, 22:129.
Crystallography of Contemporary Contact Insecticides
NYU investigators previously discovered that the efficacy of crystalline contact insecticides depends on the crystalline form (a.k.a. polymorphs). Polymorphism in contact insecticides, however, and its importance to efficacy, was largely unknown to the vector control community. Crystallographic characterization of contact insecticide solids needs to be systematic to identify more active solid forms, particularly because insecticides continues to be compromised by insecticide resistance, especially among Anopheles mosquito populations that spread malaria. Although insecticidal compounds with new modes of action have been introduced to overcome resistance, new insecticides are expensive to develop and implement. The repurposing of existing chemical agents in metastable, more active crystalline forms provides an inexpensive and efficient method for ‘evergreening’ compounds whose risks are already well-established. Here, the NYU team reports seven new crystal structures, mostly pyrethroid insecticides recommended by the WHO for indoor residual spraying, as well as a new form of a neonicotinoid insecticide.
Read the article here.
B. Erriah, X. Zhu, C.T. Hu, B. E. Kahr, A. Shtukenberg, M. D.Ward, Insects 2022, 13, 292.
Ditch the mosquitoes, keep the bees
MDI Investigators discover seven new crystal polymorphs of imidacloprid, the world’s leading insecticide,, adding to two known forms. Anticipating that insect uptake of imidacloprid molecules would depend on the respective free energies of crystal polymorph surfaces, measurements of insect knockdown times for the metastable crystal forms were as much as nine times faster acting than the commercial form against Aedes, Anopheles, and Culex mosquitoes as well as Drosophila (fruit flies). Their results suggest that replacement of commercially available imidacloprid crystals (a.k.a. Form I) in space-spraying with any one of three new polymorphs, Forms IV, VI, IX, would suppress vector-borne disease transmission while reducing environmental exposure and harm to nontarget organisms.
Read the article here.
X. Zhu, C. T. Hu, Bryan Erriah, L. Vogt-Maranto, J. Yang, Y. Yang, M. Qiu, N. Fellah, M. E. Tuckerman, M. D. Ward, B. Kahr, J. Am. Chem. Soc. 2021, 143, 17144−17152.
A deltamethrin crystal polymorph for more effective malaria control
Pyrethroid contact insecticides are mainstays of malaria control, but their efficacies are declining due to widespread insecticide resistance in Anopheles mosquito populations, a major public health challenge. Several strategies have been proposed to overcome this challenge, including insecticides with new modes of action. New insecticides, however, can be expensive to implement in low-income countries. Here, we report a simple and inexpensive method to improve the efficacy of deltamethrin, the most active and most commonly used pyrethroid, by more than 10 times against Anopheles mosquitoes. Upon heating, commercially available deltamethrin crystals, form I, melt and crystallize upon cooling into a new, kinetically stable polymorph, form II, which is much faster acting against fruit flies and mosquitoes. Epidemiological modeling suggests that the use of form II in indoor residual spraying in place of form I would significantly suppress malaria transmission, even in the presence of high levels of resistance.
Read the article here.
J. Yang, B. Erriah, C. T. Hu, E. Reiter, X. Zhu, I. P. Carmona Sepúlveda, V. López-Mejías, M. D. Ward, B. Kahr, Proc. Nat. Acad. Sci., USA, 2020, 117, 26633-26638.
Manipulating Solid Forms of Contact Insecticides for Infectious Disease Prevention
Malaria control is under threat by the development of vector resistance to pyrethroids in long-lasting insecticidal nets, which has prompted calls for a return to the notorious crystalline contact insecticide DDT. A faster acting difluoro congener, DFDT, was developed in Germany during World War II, but in 1945 Allied inspectors dismissed its superior performance and reduced toxicity to mammals. Herein, we report the discovery of amorphous and crystalline forms of DFDT and a monofluorinated chiral congener, MFDT. These solid forms were evaluated against Drosophila as well as Anopheles and Aedes mosquitoes, the former identified as disease vectors for malaria and the latter for Zika, yellow fever, dengue, and chikungunya. Contact insecticides are transmitted to the insect when its feet contact the solid surface of the insecticide, resulting in absorption of the active agent. Crystalline DFDT and MFDT were much faster killers than DDT, and their amorphous forms were even faster. The speed of action (a.k.a. knockdown time), which is critical to mitigating vector resistance, depends inversely on the thermodynamic stability of the solid form. Furthermore, one enantiomer of the chiral MFDT exhibits faster knockdown speeds than the other, demonstrating chiral discrimination during the uptake of the insecticide or when binding at the sodium channel, the presumed destination of the neurotoxin. These observations demonstrate an unambiguous link between thermodynamic stability and knockdown time for important disease vectors, suggesting that manipulation of the solid-state chemistry of contact insecticides, demonstrated here for DFDT and MFDT, is a viable strategy for mitigating insect-borne diseases, with an accompanying benefit of reducing environmental impact.fast-acting crystalline and amorphous forms of contact insecticides against Drosophila and Anopheles and Aedes mosquitoes, linking their effectiveness to the stability of the solid forms while rediscovering a compound that was developed in Germany during World War II, but vanished from public health considerations.
Read the article here.
X. Zhu, C. T. Hu, J. Yang, L. A. Joyce, M. Qiu, M. D. Ward, B. Kahr, J. Am. Chem. Soc. 2019, 14, 16858-16864.
Also see NY Times Science Times.
Inverse Correlation between Lethality and Thermodynamic Stability
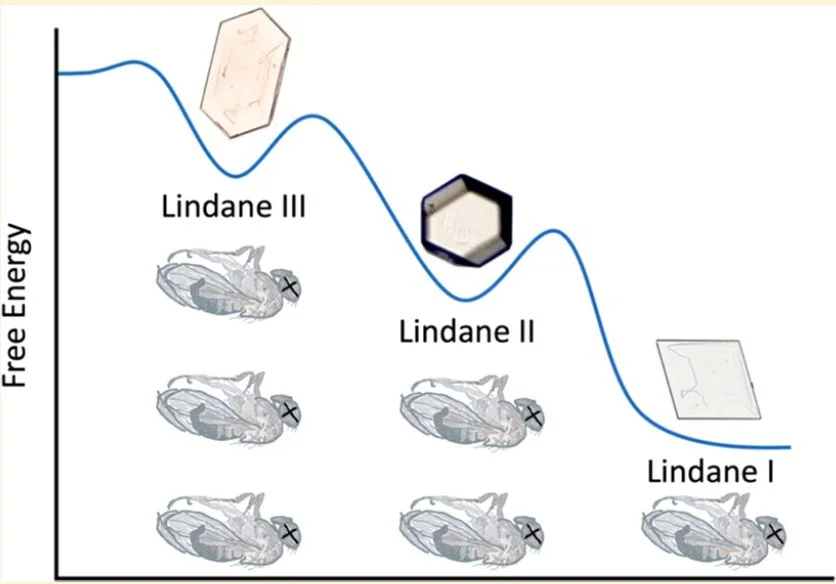
Contact insecticides often involve the interaction of whole organisms with their crystal surfaces. Lindane (1R,2r,3S,4R,5r,6S-hexachlorocyclohexane) has been one of the most widely used insecticides, but other (inactive) stereoisomers accompanying its manufacture have led to massive chemical waste remediation problems. Only one crystal structure of lindane has been reported. Herein, we report the discovery and characterization of two new polymorphs, Forms II and III. The efficacy of Forms I, II, and III against Drosophila melanogaster revealed an inverse correlation between lethality and thermodynamic stability of the polymorphs; the least stable kills fastest. This suggests a crystal engineering opportunity wherein formulations containing the most active contact insecticide polymorph can achieve infectious disease prophylaxis while reducing environmental exposure and associated chemical waste.
Read the article here
1J. Yang, X. Zhu, C. T. Hu, M. Qiu, Q. Zhu, M. D. Ward, B. Kahr. Cryst. Growth Des. 2019, 19, 1839 – 1844.
DDT Polymorphism and the Lethality of Crystal Forms
DDT (1,1,1-trichloro-2,2-bis(4-chlorophenyl)-ethane), a contact insecticide with a rich and controversial history since its activity was discovered in 1939, has long been thought to be monomorphic. Herein we report the discovery and characterization of a second polymorph, designated Form II, which can be isolated as single crystals, but converts very slowly at room temperature to the form reported previously, now designated as Form I. Computations based on an evolutionary algorithm for crystal structure prediction revealed that Forms I and II are among the four lowest energy crystal structures of fifty calculated. A preliminary study of the contact insecticidal activity toward fruit flies (Drosophila melanogaster) indicates that Form II is more active, suggesting opportunities for more effective solid-state formulations that would allow reduced amounts of DDT, thereby minimizing environmental impact.DDT (1,1,1-trichloro-2,2-bis(4-chlorophenyl)- ethane), a contact insecticide with a rich and controversial history since its activity was discovered in 1939, has long been thought to be monomorphic. Herein we report the discovery and characterization of a second polymorph, designated Form II, which can be isolated as single crystals, but converts very slowly at room temperature to the form reported previously, now designated as Form I. Computations based on an evolutionary algorithm for crystal structure prediction revealed that Forms I and II are among the four lowest energy crystal structures of fifty calculated. A preliminary study of the contact insecticidal activity toward fruit flies (Drosophila melanogaster) indicates that Form II is more active, suggesting opportunities for more effective solid-state formulations that would allow reduced amounts of DDT, thereby minimizing environmental impact.
Read the article here.
J. Yang, C. Hu, X. Zhu, Q. Zhu, M. D. Ward, B. Kahr, Angew. Chem. Int. Ed. 2017, 129,10299 –10303.