Single Crystal Structures of Contact Insecticides
NYU DMREF Investigators have discovered numerous polymorphs of contact insecticides since the initial discovery of Form II of DDT in 2017. Below is a list of these polymorphs with links to their Crystallographic Information Files (CIFs) if single crystal structures have been determined. Click on the polymorph to download the corresponding CIF, which can be view conveniently in Mercury (free download from the CCDC. The corresponding Cambridge Structural Database Refcodes also are provided for structures deposited in the CSD.
Compound Name and CSD Refcode
DDT (1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane)
DDT Polymorphism and the Lethality of Crystal Forms,
Angew. Chem. 2017, 129, 10299–10303 (DOI: 10.1002/anie.201703028)
DDT Form I (previously reported, redeterrmined, CSD Refcode: CPTCET12)
DDT Form II (NYU, CSD Refcode: CPTCET11)
DFDT (1,1,1-trichloro-2,2-bis(4-fluorophenyl)ethane)
Manipulating Solid Forms of Contact Insecticides for Infectious Disease Prevention,
J. Am. Chem. Soc. 2019, 141, 16858−16864 (DOI: 10.1021/jacs.9b08125)
DFDT Form I (NYU, CSD Refcode: GOXCUU)
DFDT Form II (NYU, CSD Refcode: GOXCUU01)
MFDT (1,1,1-trichloro-2,2-(4-chlorophenyl)-(4-fluorophenyl)-ethane)
Manipulating Solid Forms of Contact Insecticides for Infectious Disease Prevention,
J. Am. Chem. Soc. 2019, 141, 16858−16864 (DOI: 10.1021/jacs.9b08125)
(RS)-MFDT Form I (NYU, CSD Refcode: GOXDEF)
(RS)-MFDT Form II (NYU, CSD Refcode: GOXDEF01)
(RS)-MFDT Form III (NYU, CSD Refcode:GOXDEF02)
(R)-MFDT (NYU, CSD Refcode: GOXDIJ)
(S)-MFDT (NYU, CSD Refcode: GOXDOP)
Lindane (1R,2r,3S,4R,5r,6S-hexachlorocyclohexane; γ-hexachlorocyclohexane)
Inverse Correlation between Lethality and Thermodynamic Stability of Contact Insecticide Polymorphs, Cryst. Growth Des. 2019, 19, 1839−1844 (DOI: 10.1021/acs.cgd.8b01800)
Lindane Form I (NYU, CSD Refcode: HCCYHG04)
Lindane Form II (NYU, CSD Refcode: HCCYHG05)
Lindane Form III (NYU, CSD Refcode: HCCYHG06)
Imidacloprid (1-[(6-chloro-3-pyridinyl)-methyl]-N-nitro-2-imidazolidinimine)
Imidacloprid Crystal Polymorphs for Disease Vector Control and Pollinator Protection,
J. Am. Chem. Soc. 2021, 143, 17144−17152 (https://doi.org/10.1021/jacs.1c07610)
IMI Form I (previously known, structure redetermined, CSD Refcode: HANFOS05)
IMI Form II (NYU, CSD Refcode:)
IMI Form III (crystal structure not determined)
IMI Form IV (NYU, CSD Refcode: HANFOS07)
IMI Form V (NYU, CSD Refcode: HANFOS08)
IMI Form VI (NYU, CSD Refcode: HANFOS09)
IMI Form VII (crystal structure not determined)
IMI Form VIII (NYU, CSD Refcode: HANFOS10)
IMI Form IX (NYU, CSD Refcode: HANFOS10)
Deltamethrin (S)-cyano(3-phenoxyphenyl)methyl(1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylate
A deltamethrin crystal polymorph for more effective malaria control,
Proc. Nat. Acad. Sci., USA, 2020, 117, 26633-26638 (www.pnas.org/cgi/doi/10.1073/pnas.2013390117)
DM Form I (previously reported, redetermined, CSD Refcode: PXBVCP11)
DM Form II (NYU, CSD Refcode: PXBVCP12)
Chlorfenapyr (4-bromo-2-(4-chlorophenyl)-1-ethoxymethyl-5-trifluoromethyl-1H-pyrrole-3-carbonitrile)
Chlorfenapyr Crystal Polymorphism and Insecticidal Activity, Cryst. Growth Des.
XXXX, XXX, XXX−XXX (https://doi.org/10.1021/acs.cgd.3c01257)
CFP Form I (NYU, CSD Refcode: BOKVAC)
CFP Form II (NYU, CSD Refcode: BOKVEG)
CFP Form III (NYU, CSD Refcode: BOKTOO)
CFP Form IV (NYU, CSD Refcode: BOKTUU)
Miscellaneous
Crystallography of Contemporary Contact Insecticides, Insects 2022, 13, 292. (https://doi.org/10.3390/insects13030292)
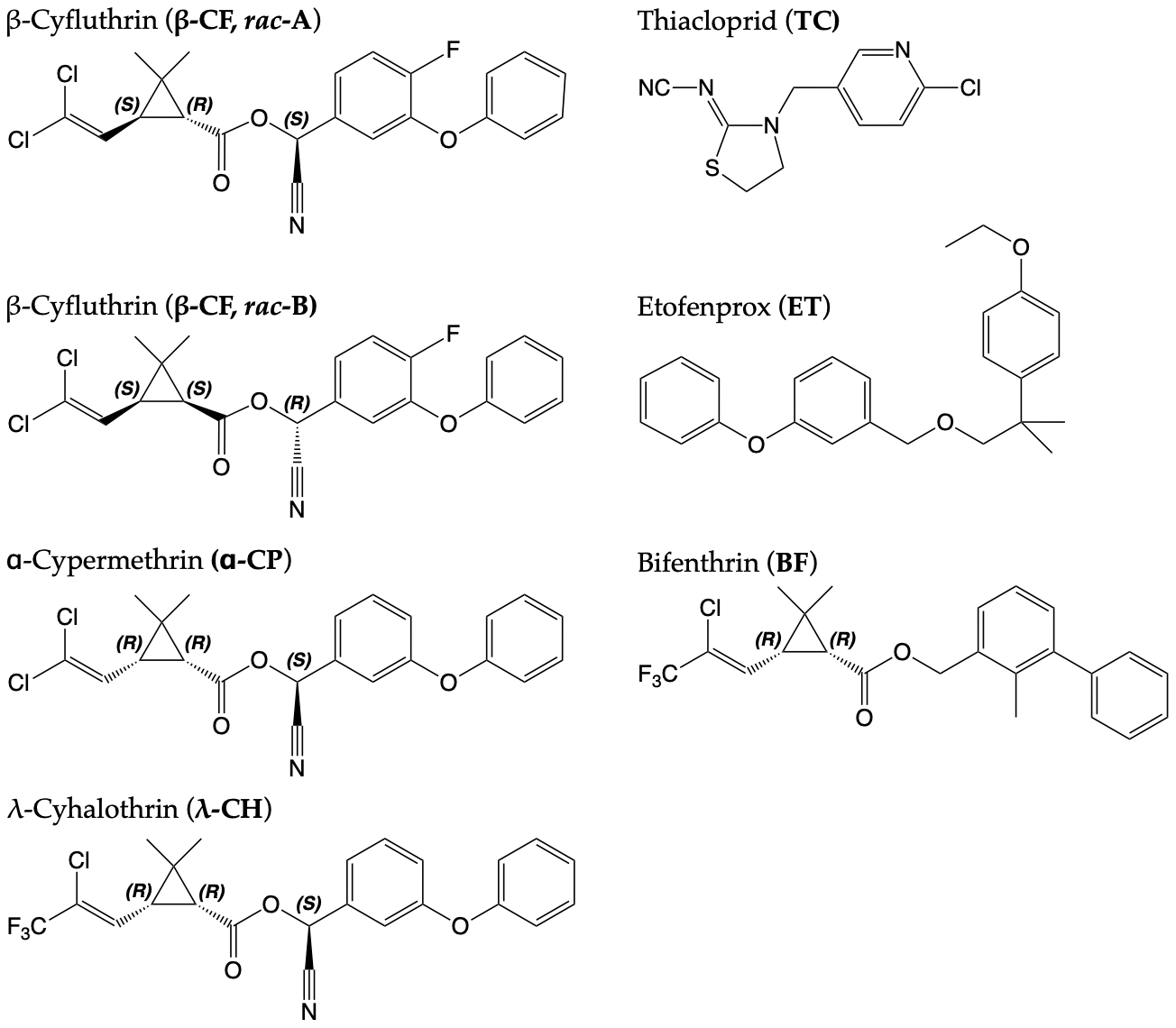
Click here for molecular structures
ɑ-Cypermethrin Form I (NYU, CSD Refcode: 2142947)
Bifenthrin Form I (NYU, CSD Refcode: 2142944)
Etofenprox Form I (NYU, CSD Refcode: 2142943)
Thiacloprid Form I (NYU, CSD Refcode: 2142940)
Thiacloprid Form II (NYU, CSD Refcode: 2142939)
β-Cyfluthrin Rac-A (NYU, CSD Refcode: 2142946)
β-Cyfluthrin Rac-B (NYU, CSD Refcode: 2142945)
λ-Cyhalothrin Form I (NYU, CSD Refcode: 2142941)
λ-Cyhalothrin Form II (NYU, CSD Refcode: 2142942)
Insecticidal and Repellent Properties of Rapid-Acting Fluorine-Containing Compounds against Aedes aegypti Mosquitoes, ACS Infect. Dis. 2023, 9, 1396−1407 (https://doi.org/10.1021/acsinfecdis.3c00161)
Click here for molecular structures
(R)-PFTE (NYU, CCDC Deposition No.: 2102296)
(S)-PFTE (NYU, CCDC Deposition No.: 2102297)
Compound 1c (NYU, CCDC Deposition No.: 2102298)
Compound 1e (NYU, CCDC Deposition No.: 2102299)
Compound 1f (NYU, CCDC Deposition No.: 2102300)
Compound 1h (NYU, CCDC Deposition No.: 2102301)
Compound 1i (NYU, CCDC Deposition No.: 2102302)
Compound 2c o,o',p,p'-TFDT (NYU, CCDC Deposition No.: 2102303)
2d o,m',p,p'-TFDT (NYU, CCDC Deposition No.: 2102304)